|
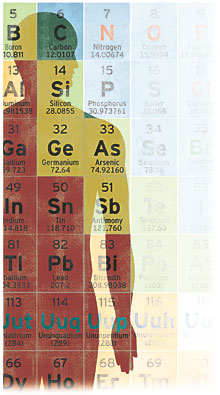
Which is true – at least the part about us being bags of water. Every school kid learns that humans are mostly water, albeit in varying amounts. The average adult is about 60 percent water. Newborns are 78 percent; obese people can be less than 50 percent water, since lean muscle tissue contains much more water (75 percent) than fat (14 percent). But as basic as water is to the human condition, other things are even more elemental, such as the hydrogen and oxygen that combine to make water. Roughly 99 percent of your body's mass is composed of just six elements: oxygen (65 percent); carbon (18 percent); hydrogen (10 percent); nitrogen (3 percent); calcium (1.5 percent); and phosphorus (1 percent). In sheer numbers of atoms, however, hydrogen tops the list. Because it's the simplest of all the elements – just one proton and one electron – hydrogen is the most abundant element in the universe. Roughly 90 percent of the atoms in the universe are hydrogen atoms. Humans follow suit. Approximately 62 percent of the atoms in every person are hydrogen atoms, followed by oxygen (24 percent); carbon (12 percent) and nitrogen (1 percent). The remaining 1 percent of the human recipe consists of tiny amounts of potassium, sulfur, sodium, chlorine and magnesium, plus even smaller traces of elements like iron, copper, molybdenum, zinc and iodine. Though they comprise less than 0.5 percent of the whole, these trace elements are just as essential as oxygen or hydrogen. You need molybdenum, for example, to activate key enzymes required for thousands of life functions. You just don't need very much. Molybdenum makes up just one atom in every billion atoms of the estimated 7,000,000,000,000,000,000,000,000,000 atoms in a typical person. A PLANET OF ONE
Even our genome isn't entirely our own: Approximately 8 percent of it is comprised of self-replicating virus-like DNA particles that infected ancient humans. THE SUM OF OUR 'SOMES
A metabolome is the complete set of metabolites found in an organism. A metabolite is any chemical or substance involved in the metabolic process, such as amino acids, sugars and fats. A 2007 Canadian draft of the human metabolome cataloged 2,500 metabolites, plus 1,500 drugs and 3,500 chemicals found in the food we eat. The human proteome remains a work in progress. Researchers estimate more than 100,000 different proteins will be found in the human body, each made up of different combinations of 20 amino acids. TRACES OF YOUAll of following elements are found in humans. In small quantities, they serve essential functions. In large quantities, they can be problematic, even deadly. The benefits and dangers of some are well known; others not so much. Arsenic (As) – Naturally occurring dietary sources of arsenic (almonds, eggs) appear to promote cell health and help displace the toxic industrial form of the mineral. Boron (B) – May promote bone and joint health, particularly in women. Calcium (Ca) – Essential to formation of bones and teeth, effective transmission of nerve impulses and muscle contractions. Chlorine (Cl) – Key to nerve function and cell cleansing. Chromium (Cr) – Important for protein synthesis, energy storage and balancing blood sugar. Cobalt (Co) – “Good” intestinal bacteria use dietary cobalt to make B-12 for your benefit. Copper (Cu) – Used in formation of bone and red blood cells; enzymes involved in healing, nerves and RNA also use it. Fluorine (F) – In natural compound form, calcium fluoride, helps develop and strengthen bones and teeth. Germanium (Ge) – Catalyst for oxygen utilization and immune function. Iodine (I) – Keeps thyroid gland healthy, helps metabolize fat and aids mental function. Iridium (Ir) – Found in brain, serves as a sort of neural superconductor. Iron (Fe) – Essential to general health and several body systems; serves as blood-oxygen transporter. Lithium (Li) – Appears to help moderate mental function and boost immune response. Magnesium (Mg) – Activator of many key enzymes; necessary for glucose metabolism. Manganese (Mn) – Helps metabolize protein and fat and maintain immune and nervous system health. Molybdenum (Mo) – Essential part of enzyme needed to convert fat to energy. Nickel (Ni) – Stabilizes DNA and RNA; important to longevity. Phosphorus (P) – Essential to virtually every body process, including cell growth, bone and tooth formation, kidney function and heart contractions. Potassium (K) – Helps regulate fluid flow in and out of cells; involved in energy storage and conversion. Rubidium (Rb) – Transporter of other minerals into cells. Selenium (Se) – An antioxidant in combination with vitamin E; supports energy production and oxygen delivery. Silicon (Si) – Required for healthy skin, ligaments, tendons and bone. Sodium (Na) – Helps balance pH in body, regulate water levels and aid nerve conduction. Strontium (Sr) – Protects energy production in cells. Sulfur (S) – Integral component of protein. Vanadium (V) – Essential to iron metabolism, red blood cell growth and cartilage health. Zinc (Zn) – Used by enzymes in digestion; DNA synthesis; involved in reproductive system. MAKE GOOD USE OF YOURSELFThe average human body contains enough sulfur to kill all of the fleas on an average-sized dog, enough carbon to make 900 pencils, enough potassium to fire a toy cannon, enough fat to make seven bars of soap, enough phosphorus to make 2,200 match heads, enough water to fill a 10-gallon container, and enough iron to make a 3-inch nail. YOU HAVE TO START SOMEWHEREMoments after the Big Bang 13.7 billion years ago, there were just two kinds of atomic nuclei in the young but fast-growing universe: hydrogen and helium (each existing in two isotopes or versions), plus a trace of lithium. For the next few million years, according to cosmologists, vast clouds of these two elements expanded outward until gravity finally caused them to collapse back into dense clumps that would become star factories. The first resulting stars were massive, tens to hundreds of times larger than the sun. These stars burned brighter than anything seen today, and died spectacularly. Their supernova deaths, hot and violent, created the conditions necessary to form other elements, most notably the stuff of life: oxygen, carbon and nitrogen. And so began a kind of cosmic chain reaction: Subsequent stars were juiced by the additional material. They burned even hotter, creating more elements in their lives and deaths. It's a process that continues, though perhaps less energetically as the universe expands, ages and cools. The last supernova seen in our galaxy (there have been others farther out) is SN1604, first described by German astronomer Johannes Kepler in 1604. Commonly called Kepler's star, it lies 20,000 light-years from Earth and was, for a time, the brightest object in the night sky. |