| System |
|
|
|
|
| English: | 1 ft = 12 in
1 mile = 5280 ft |
1 gal = 4 qt
1 qt = 2 pints 1 pt = 16 fl oz |
1 lb = 16 oz
1 ton = 2000 lb |
|
| SI-
English: |
2.54 cm = 1 in
1.609 km = 1 mi |
0.946 L = 1 qt
3.785 L= 1 gal 29.57 mL = 1 fl oz. |
453.6 g = 1 lb
28.35 g = 1 oz 1 kg = 2.205 lb |
|
| Misc.info |
|
|
||
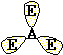 Trigonal
Trigonal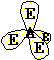 Tetrahedral
Tetrahedral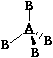 Tetrahedral
Tetrahedral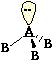 Pyramidal
Pyramidal Bent
Bent