12
VARIATIONS OF BASIC AA AND FP
The Graphite Furnace Atomizer
A non-flame type of atomizer has been found acceptable for AA units and indeed offers some advantages. This is a small high-temperature furnace known as a graphite furnace. There are several different designs, but basically this furnace is a small cylindrically shaped furnace with a sample injection port at the top. The light to be absorbed enters one end of the cylinder and emerges through the other end. The sample solution (from 1-100 uL) is syringe-injected into the furnace through the injection port. The high temperature of the furnace (about 2500oC) is reached in stages, ultimately resulting in atomization as in the flame. The atomized metal species then absorbs the light, and the absorption is measured.
One obvious difference between the furnace and the flame is that, contrary to the flame, the sample is not continuously fed into the furnace and the sample distribution is neither homogeneous nor reproducible. Thus, a furnace offers greater sensitivity (because more atoms can be placed in the path of the light) and requires less sample, but sometimes suffers from lack of accuracy and precision. Thus, the graphite furnace should be used only when the sample size is small and/or when the greater sensitivity is needed.
Inductively Coupled Plasma (ICP)
One atomic emission method that has received a great deal of attention recently is the Inductively Coupled Plasma method, better known as the ICP. As the name implies, an inductively coupled plasma is the source of the emission. This source consists of an induction coil and a plasma.
An induction coil is a coil of wire that has an alternating (oscillating) current flowing through it. Quite simply, this current induces a magnetic field around and especially inside the coil, which can be quite strong under the right conditions, coupling a great deal of energy to charged particles inside the coil.
In the ICP source, this coil is wrapped around a quartz tube through which flows a "plasma." A plasma is a collection of charged particles capable, by virtue of their charge, of interacting with a magnetic field.
Specifically, in this case, the plasma consists of a stream of argon gas that has been partially ionized by a "Tesla" coil prior to entering the tube. The interaction of the induced magnetic field with the argon plasma produces more argon ionizations and an extremely hot flame-like emission that is the ICP. With respect to the measurement of sample solutions, the procedure is an aspiration procedure, similar to FP and flame AA, in which the solution is aspirated into the flowing argon prior to entering the quartz tube. The net result is an extremely high temperature (9,000-10,000 K) "flame" that is capable of producing very intense emissions from atomized and excited atoms from the sample solution.
Being an emission technique, it is very useful for qualitative analysis, especially given the greater intensity of emission lines compared to FP. However, its major advantages lie in quantitative analysis. The linear range for I vs. c plots is much greater and thus sample solutions can be analyzed accurately over broader concentration ranges. In addition, given the increase in the emission intensity at the higher temperature, the sensitivity is much greater. Finally, simultaneous "multielement" analysis of one sample is possible. A disadvantage, perhaps, is the high cost of the equipment compared to AA and FP.
Atomic Fluorescence
When atoms that have been elevated to higher energy levels return to the ground state, the pathway could take them to some intermediate electronic states prior to the final drop. Such a series of drops back to the ground state, if accompanied by light emission, is a form of fluorescence. We can it atomic fluorescence. As with molecular fluorescence, the intensity of this emitted light is measured at right angles to the incident light and related to concentration.
Are or Spark Emission Spectrography
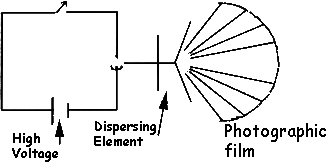
The final atomic technique we will mention is spark or arc emission spectrography. In this technique, a high voltage is used to excite a solid sample held in an electrode in such a way that when a spark jumps from this electrode to another electrode in the arrangement, atomization, excitation, and emission occur, and the emitted light again is measured. The usual configuration is such that the emitted light is dispersed and then detected with the use of photographic film. The "picture" that results is that of a combined line spectrum of all the elements in the sample. Identification (qualitative analysis) is then possible by comparing the locations of the lines on the film to the locations of lines on a standard film. Figure 17 shows the instrumental arrangement.