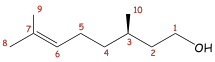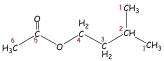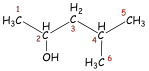| INTERPRETING
2D NMR SPECTRA1
|
||||||||
How To Read COSY Spectra
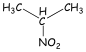
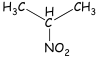
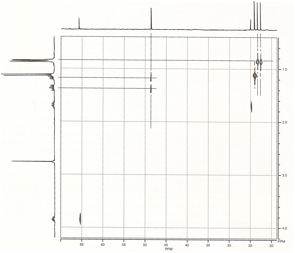
2-Nitropropane: To see what type of information a COSY
spectrum may provide. we shall consider several examples of increasing
complexity. The first is the COSY spectrum of 2-nitropropane. In this simple
molecule, we expect to observe coupling between the protons on the two methyl
groups and the proton at the methine position.
The
diagonal peaks serve only as reference points. The important peaks in the
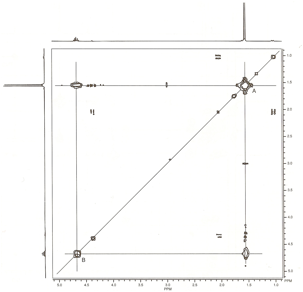
spectrum are the oil-diagonal peaks. In the spectrum of 2-nitropropane, we can
extend a horizontal line from the spot at 1.56 ppm (which is labeled A and
corresponds to the methyl protons). This horizontal line eventually
encounters an off-diagonal spot (al the upper left of the COSY spectrum) that
corresponds to the methine proton peak at 4.66 ppm (labeled B). A vertical line
drawn from this off diagonal spot intersects the spot on the diagonal that
corresponds to the methine proton (B). The presence of this off-diagonal spot,
which correlates the methyl proton spot and the methine proton spot, confirms
that the methyl protons arc coupled to the methine protons. as we would have
expected. A similar result would have been obtained by drawing a vertical line
from the 1.56-ppm spot (A) and at horizontal line from the 4.66-ppm spot (B).
The two lines would have intersected at the second off-diagonal spot (at the
lower right of the COSY spectrum). The vertical and horizontal lines described
in this analysis are drawn on the COSY spectrum in Figure 1.
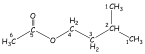
Isopentyl Acetate. In
practice, we would not
require a COSY spectrum to fully interpret the NMR spectrum of 2-nitropropane.
The preceding analysis illustrated how to interpret a COSY spectrum, using a
simple. easy-to-understand example. A more interesting example is the COSY spectrum
of isopentyl acetic (Fig. 2).
You may have
noticed that each of the COSY spectra shown in this section contains additional
spots besides the ones examined in our discussion. Often these
"extra" spots have much lower intensities than the principal spots on
the plot. The COSY method can sometimes detect interactions between nuclei over
ranges that extend beyond three bonds. Besides this long-range coupling, nuclei
that are several atoms apart but that are close together spatially also
may produce off-diagonal peaks. We learn to ignore these minor peaks in our
interpretation of COSY spectra. In
some variations of the method, however, spectroscopist make use of such
long-range interactions to produce two-dimensional NMR spectra that
specifically record this type of information.
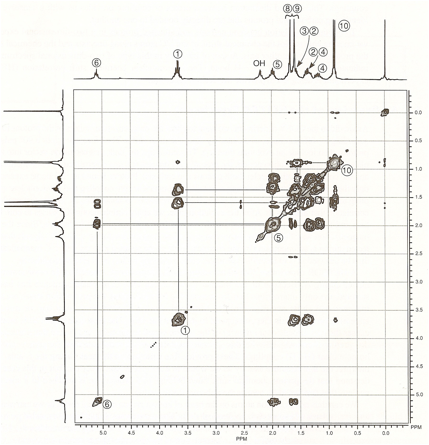
Citronellol. The COSY spectrum of citronellol is a
third example. The spectrum (Figure 3) is rather complex in appearance. Nevertheless,
we can identify certain important coupling interactions. Again, lines have been
drawn to help you identify the correlations. The proton on C6 is clearly coupled to the protons on C5. Closer examination of the spectrum also
reveals that the proton on C6 is
coupled through allylic (four-bond) coupling to the two methyl groups at C8 and C9. The protons on C1 are coupled to two nonequivalent protons
on C2 (at 1.4 and 1.6 ppm). They are
nonequivalent, owing to the presence of a stereocenter in the molecule at C3. The splitting
of the methyl protons at C10 by the methine
proton at C3 can also be seen. although the C3 spot on the diagonal line is obscured by
other spots that
Figure 3 COSY spectrum of citronellol
How To Read HETCOR Spectra
If you draw a vertical line from the methyl peak of the carbon
spectrum (21 ppm) and a horizontal line from the methyl peak of the proton
spectrum (1.56 ppm), the two lines would
intersect at the exact point on the two-dimensional plot where a spot is
marked. This spot
indicates that the protons at 1.56 ppm and the carbons at 21 ppm represent the
same position of the molecule. That is, the hydrogens are attached to the
indicated carbon. In the same way, the spot in the lower left corner of the
HETCOR plot correlates with the carbon peak at 79 ppm and the proton septet at
4.66 ppm, indicating that these two absorptions represent the same position in
the molecule.
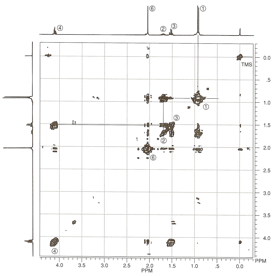
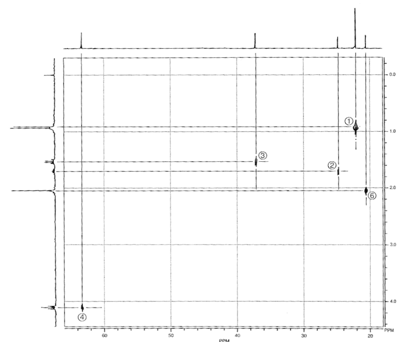
Isopentyl Acetate. A
second, more complex example is isopentyl acetate. Figure 5 is the HETCOR plot
for this substance. Each spot on
the HETCOR plot has been labeled with a number and lines have been drawn to
help you see the correlations between proton peaks and carbon peaks. The carbon
peak at 23 ppm and the proton doublet at 0.92 ppm correspond to the methyl
groups (1); the carbon peak at 25 ppm and the proton multiplet at 1.69 ppm
correspond to the methine position (2); and the carbon peak at 37 ppm and the
proton quartet at 1.52ppm correspond to the methylene group (3). The other methylene
group (4) is deshielded by the nearby oxygen atom. Therefore, a spot on the
HETCOR plot for this group appears at 63 ppm on the carbon axis and 4.10 ppm on
the proton axis. It is interesting that the methyl group of the acetyl function
(6) appears down field of the methyl groups of the isopentyl group (1) in the
proton spectrum (2.04 ppm). We expect this chemical shift, since the methyl
protons should be deshielded by the
Figure 5.
HETCOR spectrum of isopentyl acetate.
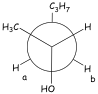
4-Methyl-2-Pentanol. Figure 6 shows
the final example that illustrates some of the power of the HETCOR technique
for 4-methyl-2-pentanol. Lines have been drawn on the spectrum to help you find
the correlations. This molecule has a stereocenter at
A great many advanced
techniques can be applied to
complex molecules. We have introduced only a few of the most
important ones here. As computers become faster and more powerful, as chemists
evolve their understanding of what different pulse sequences can achieve. and
as scientists write more sophisticated computer programs to
control those pulse sequences
and treat data, it will become possible to apply NMR
spectroscopy to
increasingly complex systems.
1. Pavia, Lampman and Kris,
Introduction to Spectroscopy, 3rd Ed. Brooks/Cole, 2001
|